PASSERIFORMES Linnaeus, 1766
There are not only more passerines than any other order of birds, there are significantly more passerines than all of the other orders put together! The Passeriformes comprise 60% of all extant bird species.
The distinguishing characteristic of the Passeriformes is the perching foot. It has an anisodactyl arrangment, with 3 toes forward, and 1 backwards. The back toe is known as the hallux. Other special characteristics of their foot include the fact that the toes join at the same level, that the toes are free to move in most passerine species, with no webbing and usually not even partially fused. Perhaps most important is their perching ability. A tendon automatically causes the foot to curl and lock in position when landing on a branch.
The passerines are often thought of as songbirds, and many are excellent songsters. This is particularly true of the oscine passerines (oscen is Latin for songbird). The oscine passerines have an especially developed syrinx that includes the lower trachea and upper bronchi. In the suboscines the syrinx is restricted to the trachea. As a result, suboscines are sometimes known as tracheophones, especially in the older literature. The New Zealand Wrens also have a tracheophone syrinx. For a long time they were considered suboscines because of this.
The Name Passeriformes
The name Passeriformes (in the form Passeres) has been attributed to Linnaeus, 1766. In the absence of a code for order-level names, I continue that tradition in the name of stability. Other choices would create nomenclatural havoc.
However, attributing Passeriformes to Linnaeus suffers from a problem. Linnaeus did not base it on a genus name he used. Indeed, he already used in in 1758. In 1766, he at least mentions the genus Passer, citing Brisson. However, he considered the House Sparrow to be Fringilla domestica, not Passer domesticus. Thus Passeres is not properly based on a genus name. Brodkorb (1978), who does attribute Passeriformes to Linnaeus, places Nitzsch (1820) next in the priority line. But I also have similar doubts about Nitzsch's use of Passer. I think strictly enforcing the requirement that the order-level name be based on one of the genera used in the text would end up giving priority to Corviformes or Hirundiformes (Wagler, 1830).
Passerine Systematics
Although we have long known which birds are passerines and which are not (the perching foot!), the relationships between them were poorly understood. Morphologically, most passerines are too similar, making it difficult or even impossible to reliably identify families and genera. DNA has changed all that!
A comparison of Clements 5th edition (which uses an old taxonomy) and the Howard-Moore 3rd and 4th editions shows how much revision has been necessary. Many passerines have been classified in the wrong family (and genus) which made it harder to determine proper family boundaries and relations. Recent work on passerine taxonomy has done much to clarify the situation, although some issues still remain.
The distinction between the oscine and suboscine passerines was pretty well sorted out in the second half of the 19th century, primarily based on the differences in the syrinx—one of the few systematic differences between passerines. Richard Bowlder Sharpe based the Catalogue of the Birds in the British Museum on his study of several of his contemporaries work on classification. The suboscines and New Zealand Wrens were grouped as Oligomyodae, and the oscine passerines as Passeres.
During the 20th century, the New Zealand Wrens were increasingly recognized as different from the suboscines, but little real progress on this was made during most of the 20th century. Even the DNA hybridization work of Sibley and Ahlquist (1990) left the question of the New Zealand Wrens unresolved. Did they belong with the suboscines, or perhaps require their own suborder? They chose the former, which was also used in the Sibley-Monroe Checklist (Sibley and Monroe, 1990; Monroe and Sibley 1993).
Finally, at the very end of the 20th century, Lovette and Bermingham (2000) sequenced part of the c-mos gene for 15 passerine genera. It was suddenly clear that Acanthisitta deserved its own suborder. This was confirmed using the RAG-1 and RAG-2 genes by Barker et al. (2002, 2004). Figure 1 of Luo et al. (2025) summarizes a number of previous estimates. I consider all of the estimates putting the origin of the in the Cretaceous to be wrong. That's everything to the left of the dashed blue vertical line in Luo et al.'s diagram.
Passerine Evolution
One question is how old are the Passeriformes? There are two ways to answer that: by looking at the split between the Passeriformes and Psittaciformes, or by looking the age of the crown group — determined by the split between Acanthisitti and the other Passeriformes.
The following tree shows now the passeriform suborders are related.

For the overall age, the respective ages from Oliveros et al. (2019), Kuhl et al. (2021) and Stiller et al. (2024) are 55.5 mya, 62.2 mya, and 61.3 mya. My guess is that the Oliveros et al. date is a bit too recent as it is very close to the radiometric age of the early passeriform fossils studied by Boles (1995). Instead, I'll use the age measured off of Fig. 2 of Luo et al. (2025), which is about 59.75 mya. Averaging, we get an estimate of 61.1 mya for the age of the split between Psittaciformes and Passeriformes.
There is considerable agreement about the age of the crown group of the Passeriformes, determined by the split between Acanthisitti and the rest of the Passeriformes. The calibrated studies by Oliveros et al. (2019), Kuhl et al. (2021), and Stiller et al. (2024) estimate the age of the Acanthisitti at 46.8 mya, 48.8 mya, and 50.6 mya, respectively. Considering the vagaries of calibration, that's pretty good agreement. We'll take it to be around 48.7 mya. It is also the age of the passeriform crown group. Regardless of when it occurred, the fossil record suggests that there was more going on then than we presently see, due to extinction of early passeriform and near-passeriform lineages.
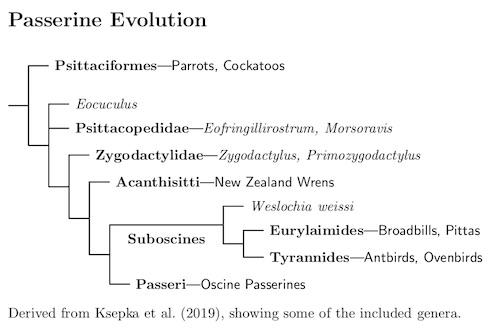
The earliest fossils that seem to belong in the passeriform crown group date from the Oligocene (the German fossil Weslochia weissi), but there are some Eocene fragments that may be stem group Passeriformes (see Mayr 2009 for more). However, there is a extinct sister group to the Passeriformes, the Zygodactylidae (Mayr, 2008b; Mayr, 2011; DeBee, 2012, and others).
Fossils of the Zygodactylidae have been found starting in the early Eocene (Green River Formation, Wyoming; Danish Fur Formation, Denmark) to the middle Miocene (France, ca. 12 mya). Based on the fossil evidence, Zygodactylidae were once one of the most abundant small birds (Mayr, 2009) and were widespread in the northern hemisphere.
Unlike modern Passeriformes, Zygodactylidae have a zygodactyl foot. This may indicate their common heritage with the zygodactyl parrots. Boletho et al. (2014), in a study of foot development, indicate how the foot transisition may have occurred. This connection, together with the fact that it can be hard to distinguish fossils of stem-parrots and stem-falcons, is why I was quick to adopt the Ericson et al. (2006a) results finding the parrots and falcons sister to the passrerines.
More recent fossil evidence has complicated matters, with the discovery of two Eocene fossils of passerine-like birds, from the Green River Formation in Wyoming and in Germany (Ksepka, Grande, and Mayr; 2019). A phylogeny based on skeletal characteristics suggest these are sister to a group that is near both the Zygodactylidae and Passeriformes—the Psittacopididae.
It has been a long process to find and sort out these and related fossils and we should not consider this the final word. It is a safe bet that further fossils will modify our views. Indeed, Ksepka, Grande, and Mayr warn that the inclusion of passeriforme-like fossil Eofringillirostrum in Psittacopididae is uncertain. If they are true passeriformes, it would cast some doubt on the idea that that passeriformes have a southern origin.
When they first started getting results from human DNA studies, they hypothesized certain migration routes based on the DNA of the people in those areas now, often using families that had lived there a while. Ancient DNA has sometimes confirmed their ideas, and sometimes refuted them. All this from a species that can't even fly over a period of mere thousands and tens of thousands of years. This makes me distrust similar speculation about birds, especially concerning events ten of millions of years ago.
One speculative alternative would be for the passerines to develop in the northern hemisphere and then move south, directly into South America for the Tyrannides, directly into Africa and Asis for the Eurylaimides. In this view, the oscines may have either separated after reaching Australasia, or separated earlier with the northern oscines wiped out in the extinction at the end of the Eocene.
New Zealand Wrens: Acanthisitti Wolters, 1977
Until recently the New Zealand wrens were considered suboscines. However, the passerines have a basal split between the New Zealand wrens and all other songbirds (Lovette and Bermingham, 2000; Barker et al., 2002; Barker et al., 2004). The common ancestor of the suboscines and the oscine passerines comes after the split between the New Zealand wrens, so we cannot put the New Zealand wrens in the suboscines. That not only forces them into their own family, but into their own suborder, Acanthisitti.
The Acanthisittidae are endemic to New Zealand. Together with the oldest splits among the suboscines and oscines, this suggest a southern origin for the Passeriform crown group.
Acanthisittidae: New Zealand Wrens Sundevall, 1872
2 genera, 4 species HBW-9
- Rifleman, Acanthisitta chloris
- Bushwren, Xenicus longipes
- New Zealand Rockwren, Xenicus gilviventris
- Stephens Island Wren, Xenicus lyalli
EUPASSERES Ericson et al., 2002b
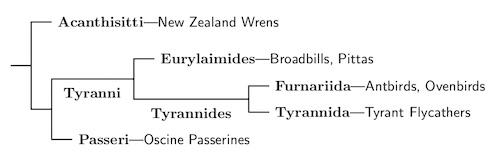
The remaining passeriformes are called the Eupasseres. They consist of the oscines (Passeri) and the suboscines (Tyranni).
Suboscines: Tyranni Wetmore & Miller, 1926
The surviving oscines have roots in Australia. The origin of the suboscines (Tyranni) has been less clear, but also appears to be in the southern hemisphere. The current topology suggests that both the Tyrannides and Eurylaimides originally diversified in South America and/or Antarctica.
It had previously been suggested that all originated when Australia, New Zealand, and Antarctica were still joined, with the ancestral Acanthisittidae in the portion that became New Zealand, the ancestral oscines in the Australian part, and the suboscines in the Antarctic part (which may have had a subtropical climate then). The western suboscines (ancestral Tyrannides) could have easily made their way to South America. The Eurylaimides remain a problem. One suggestion is that the eastern suboscines spread onto the now-submerged Kerguelen Plateau, and thence to India (see Moyle et al., 2006a). They could then ride along as India drifted into Asia.
The use of vicariance for understanding bird evolution has not been terribly reliable. It's important to recall that birds can fly.
To order the Passeriformes, the oscine group is bigger, so we consider it the main trunk, and investigate the smaller suboscine branch first. The Tyranni consists of two parts, the Old World Eurylaimides and the New World Tyrannides.
Old World Suboscines: Eurylaimides Seebohm, 1890
 |
| Click for Eurylaimides tree |
|---|
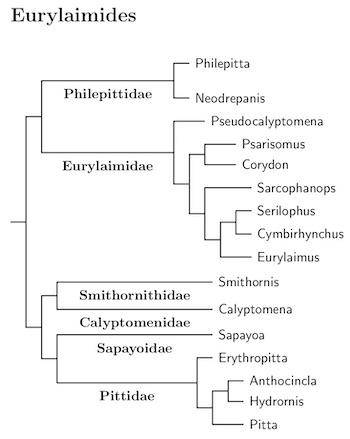
Like the passerines as a whole, the suboscines have generally been identifiable as suboscines, but teasing out the relationships between the suboscines has been a protracted process. That has changed with the publication of Harvery et al. (2020). Their analysis is based on genetic data from 1940 individuals of 1287 species (their count, not mine). Not only did they analyze a lot of individuals, they used a lot of data, from 2389 regions focusing on ultraconserved elements and exons. This is by far the most comprehensive study of the suboscines available, and they have worked hard to identify and overcome sequencing issues. Except for a few taxa that they do not include, I have followed their tree.
Beginning with the 2021 editions of these pages, I'm using the high-resolution tree from Michael Harvey's website to order the Tyranni. This is a version of Figure 1 from Harvey et al. (2020). Almost all suboscines were included in their analysis. With some help from other sources that include many of the excluded taxa, I have been able to construct an almost fully resolved tree of the Tyranni. It's long, and will be presented in pieces. The Eurylaimides are here.
Within the suboscines, the next division is between the Old World subsocines plus Sapayoa (Eurylaimides) and the New World suboscines (Tyrannides). This happened 38 mya according to Oliveros et al. (2019) and a bit over 42 mya according to Harvey et al. (2020). The arrangement of the Old World suboscines, the pittas asities, and broadbills, and Sapayoa now follows Oliveros et al. (2019) and Harvey et al. (2020). There is still some residual uncertainty about the placement of a few families, but it mostly seems pretty solid.
The Sapayoa, Sapayoa aenigma, has found a new home in this group as the only New World representative of the Eurylaimides (see Fjeldså et al., 2003; Chesser, 2004). Oliveros et al. (2019) and Harvey et al. (2020) seem to have pinned down where it belongs, as sister to the Pittidae.
Previous efforts, generally involving fewer taxa and genes, did not attain consensus on the position of the Sapayoa. These include Selvatti et al. (2017); Fjeldså et al. (2003); Chesser (2004); Irestedt et al. (2006b); Moyle et al. (2006a).
Moyle et al. (2006a) found that the broadbills were not a natural grouping. Some are more closely related to the asities than they are to the other broadbills. This list considers the broadbills to consist of three families, one of them sister to the asities, the other two are sister to each other, and then to the Pittidae. The list starts with the asities.
Philepittidae: Asities Sharpe, 1870
2 genera, 4 species HBW-8
The Asities of Madagascar are also placed in their own family. Prum et al. (2015) found that the division between the Philepittidae and Eurylaimidae dates to almost 20 mya, while Selvatti et al. (2017) placed it around 22 mya. Oliveros et al. (2019) was in-between. All agree the split was in the early Miocene. In contrast, Harvey et al. (2020) put it at 29 mya, in the Oligocene. Selvatti et al. also found that the two subfamilies of Philepittidae split about 16 mya while Harvey et al. put it just over 20 mya.
Philepittinae: Asities Sharpe, 1870
- Velvet Asity, Philepitta castanea
- Schlegel's Asity, Philepitta schlegeli
Neodrepaninae: Sunbird-Asities Shelly, 1880
- Common Sunbird-Asity, Neodrepanis coruscans
- Yellow-bellied Sunbird-Asity, Neodrepanis hypoxantha
Eurylaimidae: Eurylaimid Broadbills Lesson, 1831
7 genera, 9 species HBW-8
Except for Grauer's Broadbill, which is African, this family is Indo-Malayan. The division between Grauer's Broadbill and the rest is fairly deep, almost 20 mya according to Selvatti et al. (2017) and around 26 mya according to Harvey et al. (2020). Grauer's has been placed in a separate subfamily and may deserve its own family.
Pseudocalyptomeninae Prum, 1993
- Grauer's Broadbill, Pseudocalyptomena graueri
Eurylaiminae Lesson, 1831
- Long-tailed Broadbill, Psarisomus dalhousiae
- Dusky Broadbill, Corydon sumatranus
- Visayan Broadbill, Sarcophanops samarensis
- Wattled Broadbill, Sarcophanops steerii
- Silver-breasted Broadbill, Serilophus lunatus
- Black-and-red Broadbill, Cymbirhynchus macrorhynchos
- Banded Broadbill, Eurylaimus javanicus
- Black-and-yellow Broadbill, Eurylaimus ochromalus
Smithornithidae: African Broadbills Bonaparte, 1853
1 genus, 3 species Not HBW Family (HBW-8:83-4)
The division between African Smithornis and Calyptomena of Sundaland is quite deep. Prum et al. (2015) estimate put it in the early-Miocene, approximately 20 million years ago, but Harvey et al. (2020) put it over 30 mya in the mid-Oligocene. It seems reasonable to put them in separate families.
- Gray-headed Broadbill, Smithornis sharpei
- Rufous-sided Broadbill, Smithornis rufolateralis
- African Broadbill, Smithornis capensis
Calyptomenidae: Asian Green Broadbills Bonaparte, 1850
1 genus, 3 species Not HBW Family (HBW-8:84-5)
Hose's Broadbill, Calyptomena hosii, has been moved to be sister to the other Calyptomena, based on Harvey et al. (2020).
- Hose's Broadbill, Calyptomena hosii
- Green Broadbill, Calyptomena viridis
- Whitehead's Broadbill, Calyptomena whiteheadi
Sapayoidae: Sapayoa Irestedt et al., 2006
1 genus, 1 species Not HBW Family (HBW-9:167)
The Sapayoa is on its own old branch in Eurylaimides, and seems to be sister to the Pittidae (Oliveros et al., 2019; Harvey et al., 2020). Oliveros et al. put the split at about 27.5 mya, while for Harvey et al. it is almost 36 mya. The Sapayoa is the only Old World Suboscine in the Neotropics. There were likely many more members of its clade, with it the only survivor.
- Sapayoa, Sapayoa aenigma
Pittidae: Pittas Swainson, 1831
4 genera, 44 species HBW-8
Pitta taxonomy is now based on Harvey et al. (2020) with some help from Irestedt et al. (2006b) for Erythropitta splits that Harvey et al. did not consider. Irestedt et al., (2006b) recommended resurrecting the genera Erythropitta and Hydrornis. Harvey et al. found a relatively deep division between the Eared Pitta, Anthocincla phayrei, and the rest of Hydrornis. The most recent common ancestor seems to have been 15 mya or so, and I have separated the Eared Pitta in Anthocincla (Blyth 1862).
I had earlier split the Red-bellied Pitta, Erythropitta erythrogaster, into Northern Red-bellied Pitta, Erythropitta erythrogaster, and Southern Red-bellied Pitta, Erythropitta macklotii
The more extensive splits suggested by Irestedt et al. (2013) have been considered by Collar et al. (2015). As a result, I've further split Northern Red-bellied Pitta, Erythropitta erythrogaster, into
- Philippine Pitta, Erythropitta erythrogaster (including yairocho), inspeculata, thompsoni, and propinqua),
- Sula Pitta, Erythropitta dohertyi,
- Sulawesi Pitta, Erythropitta celebensis,
- Sangihe Pitta, Erythropitta caeruleitorques,
- and Siau Pitta, Erythropitta palliceps.
Moreover, Southern Red-bellied Pitta, Erythropitta macklotii, has been split into
- South Moluccan Pitta, Erythropitta rubrinucha (including piroensis),
- North Moluccan Pitta, Erythropitta rufiventris (including cyanonota, bernsteini, and obiensis),
- Louisiade Pitta, Erythropitta meeki,
- Papuan Pitta, Erythropitta macklotii (including finschii, aruensis, kuehni, loriae, digglesi, and oblita),
- and Bismarck Pitta, Erythropitta novaehibernicae (including extima, splendida, and gazellae).
I'm not entirely persuaded by these splits. The DNA differences found by Irestedt et al. (2013) are not strong enough to insist on species status. Collar et al.'s (2015) analysis mostly relies on plumage differences, some of them subtle. Finally, Rheindt et al. (2010) pointed to the lack of vocal differences between Sula Pitta, Erythropitta dohertyi, and Philippine Pitta, Erythropitta erythrogaster. Given the importance of vocal differences for most suboscines, one really has to wonder whether these truly represent different species.
Based on Rheindt and Eaton (2010), the Banded Pitta, Hydrornis guajanus is split into three species: Malayan Banded-Pitta, Hydrornis irena, Bornean Banded-Pitta, Hydrornis schwaneri, and Javan Banded-Pitta, Hydrornis guajanus. Since these are allopatric taxa, it is difficult to establish appropriate species limits. In my mind, the fact that irena seems to cross water barriers that are comparable to those separating the other two species suggests that more than water separates them, that they are biological species.
Finally, Yue et al. (2020) split the Ornate Pitta, Pitta concinna, and Banda Sea Pitta, Pitta vigorsii, from Elegant Pitta, Pitta elegans. Per Erritzoe and de Juana (2020), hutzi is merged into concinna.
- Blue-banded Pitta, Erythropitta arquata
- Garnet Pitta, Erythropitta granatina
- Graceful Pitta, Erythropitta venusta
- Black-crowned Pitta, Erythropitta ussheri
- Whiskered Pitta, Erythropitta kochi
- Philippine Pitta, Erythropitta erythrogaster
- Sula Pitta, Erythropitta dohertyi
- Sulawesi Pitta, Erythropitta celebensis
- Sangihe Pitta, Erythropitta caeruleitorques
- Siau Pitta, Erythropitta palliceps
- South Moluccan Pitta, Erythropitta rubrinucha
- North Moluccan Pitta, Erythropitta rufiventris
- Louisiade Pitta, Erythropitta meeki
- Papuan Pitta, Erythropitta macklotii
- Bismarck Pitta, Erythropitta novaehibernicae
- Eared Pitta, Anthocincla phayrei
- Giant Pitta, Hydrornis caeruleus
- Blue-rumped Pitta, Hydrornis soror
- Blue-naped Pitta, Hydrornis nipalensis
- Rusty-naped Pitta, Hydrornis oatesi
- Schneider's Pitta, Hydrornis schneideri
- Blue Pitta, Hydrornis cyaneus
- Bar-bellied Pitta, Hydrornis elliotii
- Gurney's Pitta, Hydrornis gurneyi
- Blue-headed Pitta, Hydrornis baudii
- Malayan Banded-Pitta, Hydrornis irena
- Bornean Banded-Pitta, Hydrornis schwaneri
- Javan Banded-Pitta, Hydrornis guajanus
- African Pitta, Pitta angolensis
- Green-breasted Pitta, Pitta reichenowi
- Indian Pitta, Pitta brachyura
- Hooded Pitta, Pitta sordida
- Mangrove Pitta, Pitta megarhyncha
- Blue-winged Pitta, Pitta moluccensis
- Fairy Pitta, Pitta nympha
- Azure-breasted Pitta, Pitta steerii
- Ornate Pitta, Pitta concinna
- Elegant Pitta, Pitta elegans
- Banda Sea Pitta, Pitta vigorsii
- Ivory-breasted Pitta, Pitta maxima
- Black-faced Pitta, Pitta anerythra
- Superb Pitta, Pitta superba
- Rainbow Pitta, Pitta iris
- Noisy Pitta, Pitta versicolor