Passeroidea
Passeroidea is the last group of families on the list. It's last mainly because of its size, over 20 families and 1500 species. The first few families involve species that have bounced around the taxonomic tree.
Promeropidae: Sugarbirds Vigors, 1825
1 genus, 2 species HBW-13
The evidence indicates these two African species are basal Passeroidea, although there is a slight chance they outside Passeroidea. Some analyses suggest they are sister to the Modulatricidae (Barker et al., 2004; Beresford et al., 2005; Fig. 3 of Johansson et al. 2008b). Others (Fjeldså et al., 2010; Fuchs et al., 2009; Fig. 2 of Johansson et al. 2008b; Nguembock et al., 2008b) find that Promerops is a separate and deeper independent branch in Passeroidea. Fjeldså et al. (2010) suggested the split might have been 45 million years ago. I think that's somewhat inflated (I don't believe the calibration), but however one dates it, it is an ancient branch. Since it is questionable whether the Promeropidae and Modulatricidae are sisters, and since the division between them is deep even if they are sisters, we treat them as separate families.
- Cape Sugarbird, Promerops cafer
- Gurney's Sugarbird, Promerops gurneyi
Modulatricidae: Spot-throat & allies Fjeldså et al. 2015
3 genera, 3 species Not HBW family
 These three birds from sub-saharan Africa have been considered babblers,
but they aren't. In fact, they've been bounced around the taxonomic tree.
Other suggestions have been that the Kakamega is a thrush, that the
Spot-throat is a bulbul or thrush, and that the Dapple-throat is a bulbul
or chat. They are none of above and belong in Passerida, next to the
sugarbirds (e.g. Johansson et al. 2008b).
These three birds from sub-saharan Africa have been considered babblers,
but they aren't. In fact, they've been bounced around the taxonomic tree.
Other suggestions have been that the Kakamega is a thrush, that the
Spot-throat is a bulbul or thrush, and that the Dapple-throat is a bulbul
or chat. They are none of above and belong in Passerida, next to the
sugarbirds (e.g. Johansson et al. 2008b).
- Spot-throat, Modulatrix stictigula
- Dapple-throat, Arcanator orostruthus
- Gray-chested Kakamega / Gray-chested Babbler, Kakamega poliothorax
Dicaeidae: Flowerpeckers Bonaparte, 1853
3 genera, 49 species HBW-13
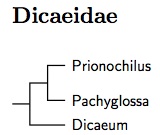 The flowerpeckers and sunbirds are sister families (Barker et al., 2004;
Ericson and Johansson, 2003, Fjeldså et al, 2010). Some merge them as Nectariniidae
(e.g., Sibley and Monroe, 1990; Christidis and Boles, 2008), but
Fjeldså et al. found a deep separation between the two clades, deeper
than that between Irenidae and Chloropseidae. Nyári et al. (2009)
sampled nearly two-thirds of the flowerpeckers. They found two major
clades, with members of Dicaeum in both clades. I've moved the
Dicaeum in the Prionochilus clade to Pachyglossa
(Blyth 1843, type melanoxantha), along with several untested species
that are likely also in that group
(vincens, annae, aeruginosa, propria).
The flowerpeckers and sunbirds are sister families (Barker et al., 2004;
Ericson and Johansson, 2003, Fjeldså et al, 2010). Some merge them as Nectariniidae
(e.g., Sibley and Monroe, 1990; Christidis and Boles, 2008), but
Fjeldså et al. found a deep separation between the two clades, deeper
than that between Irenidae and Chloropseidae. Nyári et al. (2009)
sampled nearly two-thirds of the flowerpeckers. They found two major
clades, with members of Dicaeum in both clades. I've moved the
Dicaeum in the Prionochilus clade to Pachyglossa
(Blyth 1843, type melanoxantha), along with several untested species
that are likely also in that group
(vincens, annae, aeruginosa, propria).
It's not entirely clear where the Olive-backed Flowerpecker, Prionochilus olivaceus, goes. The Bayesian and maximum likelihood trees in Nyári et al. put it sister to Pachyglossa and the remaining Prionochilus, respectively. That it is basal in the clade cannot be ruled out either. I'm treating it as the basal member of Prionochilus, but it may need its own genus.
Within Dicaeum proper, the anthonyi-quadricolor group is the basal group. The next branch is likely aureolimbatum through retrocinctum, followed by the sister species nigrilore and hypoleucum.
The remaining Dicaeum form a well-supported clade which appears to consist of three subclades: pygmaeum-virescens, sanguinolentum-maugei, and celebicum-tristrami. Note that the Mistletoebird does not appear to be that closely related to the sanguinolentum, even though they have sometimes been regarded as conspecific.
Within the celebicum-tristrami clade, the sister species celebicum and kuehni are basal, followed by another pair, ignipectus and monticolum. Interestingly, all four of these have also been considered conspecific with the Mistletoebird (hirundinaceum). Nonetheless, they also don't seem to be so closely related. Rather, the Mistletoebird seems to be more closely related to the remaining species, and particularly the Red-capped Flowerpecker (geelvinkianum). Only five of the eleven species in the hirundinaceum-tristrami group were sampled, and except for the Mistletoebird, I've adopted the arrangement in HBW, which is consistent with the maximum likelihood tree of Nyári et al. (2009).
The Wakatobi Flowerpecker, Dicaeum kuehni, has been split from Gray-sided Flowerpecker, Dicaeum celebicum, based on Kelly et al. (2014). Splitting allopatric taxa is always tricky as interbreeding potential can often be only indirectly assessed. The two differ in plumage, and mitochondrial DNA differs by over 2.5% (2.53-2.83% for COI). The DNA separation is in a range that offen indicates species status. Moreover, there are noticable plumage and morphological differences as documented by Kelly et al., (2014). To me, this combination justifies the split.
Interestingly, only about 25 miles of open water separates Sulawesi from the Waktobi Islands (part of the Tukangbesi archipelago). The two have never been connected by a ice age land bridge as the water between is a mile or more deep (Google Earth). Kelly et al. found no evidence of recent gene flow in the mitochondrial DNA (COI and ND3). We don't know if any males cross the water and interbred, but it appears no females have recently done so.
Note that I do not make this split based on Hebert et al.'s (2004) barcoding threshold of 2.7%, which I find totally unconvincing. I don't think the authors then had a good understanding of the two separate questions involved: use of barcoding to identify taxa, and use of barcoding to determine species status. Certaintly the sampling and primitive statistical methods used were inadequate to the species status task. This becomes clear from more recent papers such as Tavares and Baker (2008), Kerr et al. (2009), Milá et al. (2012).
Tavares and Baker (2008), who sampled more relevant taxa, found an overlap zone of about 0.8-3.0%. Kerr et al. (2009) studied a large sample of palearctic birds. They looked at type I (false positives) and type II errors (false negatives) from using the barcoding data. They claim an optimum value of 1.6%, although it is rather unclear why this is optimal. Eyeballing their graph (Figure 2) suggests that the minimum total error is at 1.4%. I did not compute the minimum RMS error, but it probably occurs little lower as the RMS criterion puts more weight on equalizing the errors. The errors seem to be equalized at about 1.1%. Taxonomic committees seem to prefer to avoid type I error, with less emphasis on type II error, implying some sort of asymmetric loss function. The optimum error in such cases could be well to right as the type I error region on Kerr et al.'s Figure 2 extends all the way to 8%. The species limits may be incorrect in some of such cases, or the genetic integrity of the species may be maintained through males making the mitochondrial estimates irrelevant (think cuckoos! Fossøy et al., 2010).
Finally, genetic distances in the Neotropics are higher (Milá et al., 2012). To what extent this indicates more actual interspecific variation, or is an artifact of undersplitting remains unclear. I suspect that all this literature could benefit from a more sophisticated approach to both sampling and statistical analysis.
- Olive-backed Flowerpecker, Prionochilus olivaceus
- Yellow-breasted Flowerpecker, Prionochilus maculatus
- Scarlet-breasted Flowerpecker, Prionochilus thoracicus
- Yellow-rumped Flowerpecker, Prionochilus xanthopygius
- Crimson-breasted Flowerpecker, Prionochilus percussus
- Palawan Flowerpecker, Prionochilus plateni
- Yellow-vented Flowerpecker, Pachyglossa chrysorrhea
- Yellow-bellied Flowerpecker, Pachyglossa melanoxantha
- Legge's Flowerpecker, Pachyglossa vincens
- Golden-rumped Flowerpecker, Pachyglossa annae
- Thick-billed Flowerpecker, Pachyglossa agilis
- Striped Flowerpecker, Pachyglossa aeruginosa
- Brown-backed Flowerpecker, Pachyglossa everetti
- Whiskered Flowerpecker, Pachyglossa propria
- Flame-crowned Flowerpecker, Dicaeum anthonyi
- Bicolored Flowerpecker, Dicaeum bicolor
- Cebu Flowerpecker, Dicaeum quadricolor
- Yellow-sided Flowerpecker, Dicaeum aureolimbatum
- Orange-bellied Flowerpecker, Dicaeum trigonostigma
- Red-keeled Flowerpecker, Dicaeum australe
- Black-belted Flowerpecker, Dicaeum haematostictum
- Scarlet-collared Flowerpecker, Dicaeum retrocinctum
- Buzzing Flowerpecker, Dicaeum hypoleucum
- Olive-capped Flowerpecker, Dicaeum nigrilore
- Pygmy Flowerpecker, Dicaeum pygmaeum
- Pale-billed Flowerpecker, Dicaeum erythrorhynchos
- Nilgiri Flowerpecker, Dicaeum concolor
- Plain Flowerpecker, Dicaeum minullum
- Andaman Flowerpecker, Dicaeum virescens
- Blood-breasted Flowerpecker, Dicaeum sanguinolentum
- Scarlet-backed Flowerpecker, Dicaeum cruentatum
- Scarlet-headed Flowerpecker, Dicaeum trochileum
- Black-fronted Flowerpecker, Dicaeum igniferum
- Blue-cheeked Flowerpecker, Dicaeum maugei
- Gray-sided Flowerpecker, Dicaeum celebicum
- Wakatobi Flowerpecker, Dicaeum kuehni
- Fire-breasted Flowerpecker, Dicaeum ignipectus
- Black-sided Flowerpecker, Dicaeum monticolum
- Mistletoebird, Dicaeum hirundinaceum
- Crimson-crowned Flowerpecker, Dicaeum nehrkorni
- Flame-breasted Flowerpecker, Dicaeum erythrothorax
- Halmahera Flowerpecker, Dicaeum schistaceiceps
- Ashy Flowerpecker, Dicaeum vulneratum
- Olive-crowned Flowerpecker, Dicaeum pectorale
- Red-capped Flowerpecker, Dicaeum geelvinkianum
- Louisiade Flowerpecker, Dicaeum nitidum
- Red-banded Flowerpecker, Dicaeum eximium
- Midget Flowerpecker, Dicaeum aeneum
- Mottled Flowerpecker, Dicaeum tristrami
Nectariniidae: Sunbirds Vigors, 1825
17 genera, 143 species HBW-13
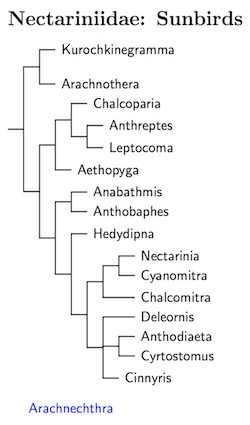 The ordering of the sunbirds is based on the tree in Jønsson and
Fjeldså (2006a), which is based on Bowie's unpublished Ph.D.
dissertation (2003). I've made two alterations, placing Chalcoparia
closer to Anthreptes and Leptocoma (see Moyle et. al, 2011),
and putting Cinnyris in a position that is a compromise between the
Bowie/Jønsson and Fjeldså tree and Nyári et al.
(2009b). Checke and Mann (2008) in HBW-13 was used to place the remaining
taxa on the resulting tree. There are two exceptions: Loten's Sunbird and
the similar Purple Sunbird don't have clear relatives and I doubt they
belong in Cinnyris, so I've moved them to the genus
Arachnechthra (Cabanis 1851, type lotenia). You'll see it
floating at the bottom of the tree.
The ordering of the sunbirds is based on the tree in Jønsson and
Fjeldså (2006a), which is based on Bowie's unpublished Ph.D.
dissertation (2003). I've made two alterations, placing Chalcoparia
closer to Anthreptes and Leptocoma (see Moyle et. al, 2011),
and putting Cinnyris in a position that is a compromise between the
Bowie/Jønsson and Fjeldså tree and Nyári et al.
(2009b). Checke and Mann (2008) in HBW-13 was used to place the remaining
taxa on the resulting tree. There are two exceptions: Loten's Sunbird and
the similar Purple Sunbird don't have clear relatives and I doubt they
belong in Cinnyris, so I've moved them to the genus
Arachnechthra (Cabanis 1851, type lotenia). You'll see it
floating at the bottom of the tree.
The basal position of the spiderhunters is clear not only from Bowie/Jønsson and Fjeldså tree and from Nyári et al. (2009b), but also from the the analysis by Moyle et al. (2011), which focuses on spiderhunters. All three sources also also have an oriental clade containing Aethopyga sister to the remaining spiderhunters.
Aethopyga has been rearranged based on Hosner et al. (2013a) and five additional endemic species from the Philippines are recognized:
- Crimson Sunbird, Aethopyga siparaja, is split from Magnificent Sunbird, Aethopyga magnifica.
- Maroon-naped Sunbird, Aethopyga guimarasensis (including daphoenonota), is split from Flaming Sunbird, Aethopyga flagrans.
- Bohol Sunbird, Aethopyga decorosa, and Luzon Sunbird, Aethopyga jefferyi, are split from Metallic-winged Sunbird, Aethopyga pulcherrima.
- Tboli Sunbird, Aethopyga tibolii, is split from Apo Sunbird, Aethopyga boltoni.
All of the new species are both genetically and visually distinct. Four have previously been considered separate species. The recently discovered A. tibolii was described as a subspecies (Kennedy et al., 1997), but it too is both genetically and visually distinctive.
Hosner et al. (2013a) also raise the issue of whether the Elegant Sunbird, Aethopyga duyvenbodei, should be placed in a separate genus (Duyvena). Their work weakly supports it as sister to the other Aethopyga, and I leave it in Aethopyga for now.
The reorganization meant subsuming Dreptes into Cinnyris and Drepanorhynchus into Cyanomitra. It also meant resolving any priority issues between Anthodiaeta and Hedydipna by recognizing both. [See Alan Peterson's analysis of the priority issue at zoonomen.net.] The four Asian and Australasian species formerly considered part of Cinnyris have been separated in their own genus, Cyrtostomus.
The genus Cinnyris is now much reduced, as it has additionally lost 15 species to Chalcomitra, 15 to Anthobaphes, and one each to Arachnechthra, Cyanomitra, and Deleornis, gaining only one species from Chalcomitra. The African members of Anthreptes have been dispersed to Deleornis (3) and Hedydipna (7). Nectarinia lost 4 species to Chalcomitra and gains two from Cyanomitra.
Orange-tufted Spiderhunter, Arachnothera flammifera (presumed to include randi), and Pale Spiderhunter, Arachnothera dilutior, have been are split from Little Spiderhunter, Arachnothera longirostra based on the analyses of Lohman et al. (2010) and Rahman et al. (2010). Between them, they consider a decent sampling of the other Little Spiderhunter subspecies, which show relatively little genetic differentiation. This is also supported by Moyle et al. (2011).
Moyle et al. (2011) also supports the split of Bornean Spiderhunter, Arachnothera everetti, from Streaky-breasted Spiderhunter, Arachnothera affinis. Although their evidence also suggests that Hypogramma should be subsumed in Arachnothera, a further analysis by Campillo et al. (2018) places Hypogramma sister to Arachnothera. However, the name Hypogramma is preoccupied and must be replaced by Kurochkinegramma (Kashin, 1978).
- Purple-naped Sunbird, Kurochkinegramma hypogrammicum
- Thick-billed Spiderhunter, Arachnothera crassirostris
- Long-billed Spiderhunter, Arachnothera robusta
- Orange-tufted Spiderhunter, Arachnothera flammifera
- Little Spiderhunter, Arachnothera longirostra
- Pale Spiderhunter, Arachnothera dilutior
- Whitehead's Spiderhunter, Arachnothera juliae
- Yellow-eared Spiderhunter, Arachnothera chrysogenys
- Naked-faced Spiderhunter, Arachnothera clarae
- Spectacled Spiderhunter, Arachnothera flavigaster
- Streaked Spiderhunter, Arachnothera magna
- Streaky-breasted Spiderhunter, Arachnothera affinis
- Gray-breasted Spiderhunter, Arachnothera modesta
- Bornean Spiderhunter, Arachnothera everetti
- Ruby-cheeked Sunbird / Rubycheek, Chalcoparia singalensis
- Plain Sunbird, Anthreptes simplex
- Brown-throated Sunbird, Anthreptes malacensis
- Gray-throated Sunbird, Anthreptes griseigularis
- Red-throated Sunbird, Anthreptes rhodolaemus
- Purple-rumped Sunbird, Leptocoma zeylonica
- Crimson-backed Sunbird, Leptocoma minima
- Purple-throated Sunbird, Leptocoma sperata
- Van Hasselt's Sunbird, Leptocoma brasiliana
- Black Sunbird, Leptocoma aspasia
- Copper-throated Sunbird, Leptocoma calcostetha
- Elegant Sunbird, Aethopyga duyvenbodei
- Fire-tailed Sunbird, Aethopyga ignicauda
- Black-throated Sunbird, Aethopyga saturata
- Mrs. Gould's Sunbird, Aethopyga gouldiae
- Green-tailed Sunbird, Aethopyga nipalensis
- Vigors's Sunbird, Aethopyga vigorsii
- Crimson Sunbird, Aethopyga siparaja
- Magnificent Sunbird, Aethopyga magnifica
- Lovely Sunbird, Aethopyga shelleyi
- Temminck's Sunbird, Aethopyga temminckii
- Javan Sunbird, Aethopyga mystacalis
- Handsome Sunbird, Aethopyga bella
- White-flanked Sunbird, Aethopyga eximia
- Fork-tailed Sunbird, Aethopyga christinae
- Flaming Sunbird, Aethopyga flagrans
- Maroon-naped Sunbird, Aethopyga guimarasensis
- Bohol Sunbird, Aethopyga decorosa
- Luzon Sunbird, Aethopyga jefferyi
- Metallic-winged Sunbird, Aethopyga pulcherrima
- Lina's Sunbird, Aethopyga linaraborae
- Gray-hooded Sunbird, Aethopyga primigenia
- Apo Sunbird, Aethopyga boltoni
- Tboli Sunbird, Aethopyga tibolii
- Reichenbach's Sunbird, Anabathmis reichenbachii
- Principe Sunbird, Anabathmis hartlaubii
- Newton's Sunbird, Anabathmis newtonii
- Orange-breasted Sunbird, Anthobaphes violacea
- Regal Sunbird, Anthobaphes regius
- Rockefeller's Sunbird, Anthobaphes rockefelleri
- Neergaard's Sunbird, Anthobaphes neergaardi
- Greater Double-collared Sunbird, Anthobaphes afer
- Miombo Double-collared Sunbird, Anthobaphes manoensis
- Southern Double-collared Sunbird, Anthobaphes chalybeus
- Northern Double-collared Sunbird, Anthobaphes reichenowi
- Ruwenzori Double-collared Sunbird, Anthobaphes stuhlmanni
- Prigogine's Double-collared Sunbird, Anthobaphes prigoginei
- Ludwig's Double-collared Sunbird, Anthobaphes ludovicensis
- Eastern Double-collared Sunbird, Anthobaphes mediocris
- Usambara Double-collared Sunbird, Anthobaphes usambaricus
- Forest Double-collared Sunbird, Anthobaphes fuelleborni
- Moreau's Sunbird, Anthobaphes moreaui
- Loveridge's Sunbird, Anthobaphes loveridgei
- Pygmy Sunbird, Hedydipna platura
- Nile Valley Sunbird, Hedydipna metallica
- Amani Sunbird, Hedydipna pallidigaster
- Plain-backed Sunbird, Hedydipna reichenowi
- Anchieta's Sunbird, Hedydipna anchietae
- Mangrove Sunbird, Hedydipna gabonica
- Western Violet-backed Sunbird, Hedydipna longuemarei
- Eastern Violet-backed Sunbird, Hedydipna orientalis
- Uluguru Violet-backed Sunbird, Hedydipna neglecta
- Violet-tailed Sunbird, Hedydipna aurantia
- Olive Sunbird, Nectarinia olivacea
- Gray Sunbird, Nectarinia veroxii
- Malachite Sunbird, Nectarinia famosa
- Scarlet-tufted Sunbird, Nectarinia johnstoni
- Golden-winged Sunbird, Cyanomitra reichenowi
- Rufous-winged Sunbird, Cyanomitra rufipennis
- Green-headed Sunbird, Cyanomitra verticalis
- Bannerman's Sunbird, Cyanomitra bannermani
- Blue-throated Brown Sunbird, Cyanomitra cyanolaema
- Cameroon Sunbird, Cyanomitra oritis
- Blue-headed Sunbird, Cyanomitra alinae
- Olive-bellied Sunbird, Chalcomitra chloropygius
- Tiny Sunbird, Chalcomitra minulla
- Malagasy Green Sunbird, Chalcomitra notata
- Violet-breasted Sunbird, Chalcomitra chalcomelas
- Pemba Sunbird, Chalcomitra pembae
- Scarlet-chested Sunbird, Chalcomitra senegalensis
- Hunter's Sunbird, Chalcomitra hunteri
- Buff-throated Sunbird, Chalcomitra adelberti
- Carmelite Sunbird, Chalcomitra fuliginosa
- Green-throated Sunbird, Chalcomitra rubescens
- Amethyst Sunbird, Chalcomitra amethystina
- Marico Sunbird, Chalcomitra mariquensis
- Purple-banded Sunbird, Chalcomitra bifasciata
- Tsavo Sunbird, Chalcomitra tsavoensis
- Shelley's Sunbird, Chalcomitra shelleyi
- Hofmann's Sunbird, Chalcomitra hofmanni
- Congo Sunbird, Chalcomitra congensis
- Red-chested Sunbird, Chalcomitra erythrocerca
- Black-bellied Sunbird, Chalcomitra nectarinioides
- Copper Sunbird, Chalcomitra cuprea
- Beautiful Sunbird, Chalcomitra pulchella
- Bocage's Sunbird, Chalcomitra bocagii
- Purple-breasted Sunbird, Chalcomitra purpureiventris
- Tacazze Sunbird, Chalcomitra tacazze
- Bronzy Sunbird, Chalcomitra kilimensis
- Fraser's Sunbird, Deleornis fraseri
- Gray-headed Sunbird, Deleornis axillaris
- Little Green Sunbird, Deleornis seimundi
- Bates's Sunbird, Deleornis batesi
- Gray-chinned Sunbird, Deleornis rectirostris
- Banded Green Sunbird, Deleornis rubritorques
- Collared Sunbird, Anthodiaeta collaris
- Olive-backed Sunbird, Cyrtostomus jugularis
- Apricot-breasted Sunbird, Cyrtostomus buettikoferi
- Flame-breasted Sunbird, Cyrtostomus solaris
- Giant Sunbird, Cinnyris thomensis
- Socotra Sunbird, Cinnyris balfouri
- Orange-tufted Sunbird, Cinnyris bouvieri
- Palestine Sunbird, Cinnyris osea
- Shining Sunbird, Cinnyris habessinicus
- Splendid Sunbird, Cinnyris coccinigastrus
- Johanna's Sunbird, Cinnyris johannae
- Superb Sunbird, Cinnyris superbus
- Oustalet's Sunbird, Cinnyris oustaleti
- White-bellied Sunbird, Cinnyris talatala
- Variable Sunbird, Cinnyris venustus
- Dusky Sunbird, Cinnyris fuscus
- Ursula's Sunbird, Cinnyris ursulae
- Seychelles Sunbird, Cinnyris dussumieri
- Humblot's Sunbird, Cinnyris humbloti
- Mayotte Sunbird, Cinnyris coquerellii
- Anjouan Sunbird, Cinnyris comorensis
- Souimanga Sunbird, Cinnyris sovimanga
- Purple Sunbird, Arachnechthra asiatica
- Loten's Sunbird, Arachnechthra lotenia
Irenidae: Fairy Bluebirds Jerdon, 1863
1 genus, 2 species HBW-10
Whether the fairy bluebirds and leafbirds of the Oriental Region should be united in the same family remains an open question. Most analyses have found they are sister families (Barker et al., 2004; Beresford et al., 2005; Fjeldså et al., 2010; Kennedy et al., 2012; Reddy and Cracraft, 2007; Moltesen et al., 2012); Treplin et al., 2008), although Barker et al. (2002) found differently and Johanssen et al. (2008b) left the issue unresolved. The recent paper by Moltesen et al. suggests that they share a common ancestor a little over 10 million years ago, making the case for treating the fairy bluebirds as a subfamily of the leafbirds. Kennedy et al. (2012) estimated that their common ancestor was likely about 20 million years ago, while Fjeldså et al. put the ancestor around 40 million years ago. In the latter case, they definitely should be separate families.
The Fjeldså et al. estimate is being driven by a single calibration point, a hypothesized origin of Acanthisitta around 82-85 million years ago. I have deep skepticism about this date, and would not be surprised if the true date were much more recent. However, I suspect Moltesen et al. gives too short a time. It seems best to leave these as separate families for now.
I'm assuming that the flowerpecker/sunbird clade is a slightly deeper branch than the fairy bluebird/leafbird clade. However, although it seems to be the consensus of the relevant papers, the overall support for this is not strong.
Moltesen et al. (2012) advocate the treatment of a number of Irena and Chloropsis subspecies as full species, primarly on the basis of genetic distance. I think they are likely right about some of them, but prefer to wait for more evidence in most cases.
- Asian Fairy-bluebird, Irena puella
- Philippine Fairy-bluebird, Irena cyanogastra
Chloropseidae: Leafbirds Wetmore, 1960 (1847)
1 genus, 12 species HBW-10
I find one of the Moltesen et al. (2012) splits more compelling as they also found that one of the subspecies was more closely related to an entirely different species. As a result, I've separated the Yellow-bordered Leafbird, Chloropsis septentrionalis from the Lesser Green-Leafbird, Chloropsis cyanopogon. There didn't seem to be a historical English name for septentrionalis, so I've given it one based on a distinguishing characteristic, the yellow border around the mask on the males, as noted by Moltesen et al.
- Blue-masked Leafbird, Chloropsis venusta
Click for Chloropseidae tree - Orange-bellied Leafbird, Chloropsis hardwickii
- Jerdon's Leafbird, Chloropsis jerdoni
- Blue-winged Leafbird, Chloropsis cochinchinensis
- Bornean Leafbird, Chloropsis kinabaluensis
- Golden-fronted Leafbird, Chloropsis aurifrons
- Greater Green-Leafbird, Chloropsis sonnerati
- Sumatran Leafbird, Chloropsis media
- Philippine Leafbird, Chloropsis flavipennis
- "Yellow-bordered Leafbird", Chloropsis septentrionalis
- Lesser Green-Leafbird, Chloropsis cyanopogon
- Yellow-throated Leafbird, Chloropsis palawanensis